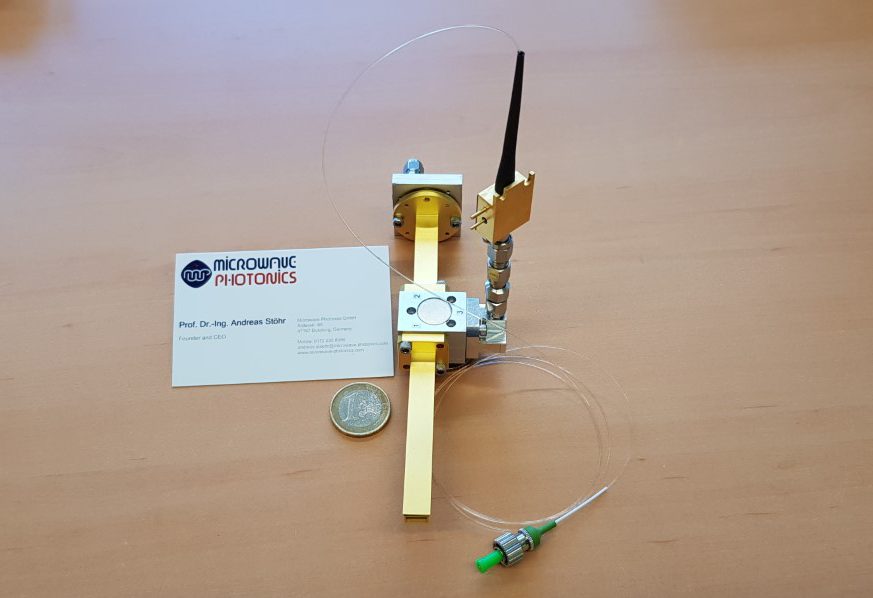
Microwave Photonics GmbH (MWP) has developed a first photonic sensor for skin cancer monitoring. The prototype is designed to support medical practitioners and surgeons in early skin-cancer detection. The development is financially supported by the European INTERREG-V-A program ROCKET Reloaded within the SkinBall project. The SkinBall project is coordinated by Microwave Photonics GmbH. The consortium also consists of Mintres BV (NL), the University Duisburg-Essen, Department ZHO/Optoelectronics (DE), and the University Hospital Essen (DE).
“Our motivation results from recent interest in monitoring various types of skin cancer by means of non-ionizing electromagnetic waves in the millimeter-wave region”, explains MWP CEO Dr. Andreas Stöhr. Previous applied research has shown that skin cancer can modify the water content of skin. This has a direct impact on the skin’s electromagnetic characteristics. Therefore, by measuring the permittivity of the skin, we aim to distinguish between healthy and non-healthy skin. Our vision is to develop a low-cost mobile sensor that can be used not only in clinics but also by normal practitioners.
Photonic sensor development
The technological challenge has been to develop a mobile sensor that operates over an extremely wide bandwidth and provides sufficient contrast for high-resolution millimeter-wave skin cancer monitoring. Facing this challenge, MWP Senior Engineer Vitaly Rymanov highlights the benefit of millimeter waves. “In particular, the millimeter waves (30-300 GHz) exhibit shorter wavelengths than microwaves and thus penetrate only a few millimeters into the human body. This makes them highly-efficient for sensing pathological variations in different skin layers, in which skin tumors primarily occur,” says Rymanov.
In the next step, the developed prototype will be validated in test laboratories. The aim is to verify the reliability in monitoring the permittivity of different biologic and non-biologic tissues. First in-vivo and in-vitro medical examinations using human skin are envisaged for the beginning of next year. If successful, we hope to provide first prototypes to clinic facilities in about 3-4 years from now.